Objectives:
- To understand that when a force causes an object to move through a distance work is done on the object. So a force does work on an object when the force causes a displacement of the object
- The work done by a force on an object can be calculated using the equation: $latex W = Fs $
- To appreciate that one joule of work is done when a force of one newton causes a displacement of one metre,
- You should be able to describe the energy transfer involved when work is done.
- You should be able to convert between newton-metres and joules.
- To understand that work done against the frictional forces acting on an object causes a rise in the temperature of the object.
You will have come across the terms and likely the equations for kinetic energy, gravitational potential energy and elastic potential energy;
Each of these describes a type of energy that we can visualise and use to our benefit in calculations. Ultimately however, they are all just ways in which energy is transferred from one location or from one object to another.
Work done is the term given to the transfer of energy and it arises from the concept of doing work on object or a system in order to lift it up, make it move faster or to even stretch it. Take lifting a crate up in the air, the crate has a mass which is effectively a measure of its resistance to motion. Because it has a resistance to motion you have to exert a force on it to make it a move through a distance – you do work on the object to move it against gravity!
By definition, when a force causes an object to move through a distance work is done on the object. The equation for work done is:
where;
is the work done, measure in joules,
is the force, measure in Newtons,
is the distance, measure in metres,
 Whenever energy is transferred to any object, whether it is to heat it up, move it, speed it up, break the bonds between the particles, stretch it etc. work is done. For a moving object, to increase its kinetic energy you need to make it move faster and as such it is not always convenient to talk about a force being exerted which is why the equation for kinetic energy does not involve a force. However, a force needs to be applied in the first place to do work on the object to speed it up so that the kinetic energy increases.
Whenever energy is transferred to any object, whether it is to heat it up, move it, speed it up, break the bonds between the particles, stretch it etc. work is done. For a moving object, to increase its kinetic energy you need to make it move faster and as such it is not always convenient to talk about a force being exerted which is why the equation for kinetic energy does not involve a force. However, a force needs to be applied in the first place to do work on the object to speed it up so that the kinetic energy increases.
By substituting the units into the equation above we get:
This tells us that is equivalent to $latex 1 \ Nm:
If you are lifting an object up, you do work against gravity and so the object gains gravitational potential energy
If you push a block along a surface horizontally you are not doing work against gravity, but you have got to apply a force or the object would just remain stationary, therefore you have to do work against the frictional forces Where physics often appears to get complicated is the fact that one thing ultimately leads to another but they can mean the same thing. In other words…
Where physics often appears to get complicated is the fact that one thing ultimately leads to another but they can mean the same thing. In other words…
Lets say I lift an apple of mass up in the air through a distance of
. The gravitational potential energy that is gained can be worked out by using
:
latex g = 9.8 \ m/s^{2} $
So the apple has gained of gravitational potential energy. This means that the word done against gravity should also equal
of energy – after all, energy is conserved and cannot be created or destroyed! Lets justify this using the definition for work done,
;
The weight of the apple if the force it acts downwards (with gravity) and therefore it is this force that we need to do work against. Weight can be determined using
We are lifting this mass through a distance of and so
:
This is the same as above – so that’s some evidence that it works.
Doing work to speed something up
When you get in a car and accelerate, the engine does work against friction to speed the car up. If a constant force, is applied over a distance
then the work done by the engine to overcome friction would be
(as explained above).
This work would go into increasing the speed of the object which inevitably means it goes into increasing the kinetic energy of the object and so we can write that . This only works of course if the work done done only goes into the kinetic energy store of the object and none is lost as heat or sound. This is very difficult to achieve but physicists like to model energy on the ‘perfect case scenario’ and so for the best part try to ignore all of these loses in energy.
Contradicting the previous paragraph, you are required to understand the process of heating up a system. This is of course achieved by doing work on the system…
What do you suppose happens if you applied work on an object but it doesn’t go anywhere? It doesn’t gain gain gravitational potential energy and it doesn’t speed up increasing its kinetic energy? Almost as though you push on a wall with all your force… You will still have supplied a force and you can feel the energy draining from your body… But where does this energy go?
The answer is similar to that of an elastic band, you stretch an elastic band and then release it, well the energy went into elastic potential and then kinetic (when you released it) but what then? Energy has to be conserved after all so it cannot just be lost?!
The energy from our point of view appears to disappear but it actually goes into the particles around it. Take the elastic band for example, you release it and the kinetic energy eventually disappears but this is because it is transferring this kinetic energy into the invisible air particles all around it when it collides with them. This is the same thing that happens when you push against a wall, the particles in the wall compress slightly, which makes them move allowing them to gain kinetic energy.
If you repeat these processes again and again the air particles will gain more and more kinetic energy. This kinetic energy spreads around the room (or object) and roughly evenly distributes itself to each air particle involved. Temperature is the term we use to describe the average kinetic energy of all the particles in a system, so if we are increasing the average kinetic energy them we effectively increase the temperature of the room!
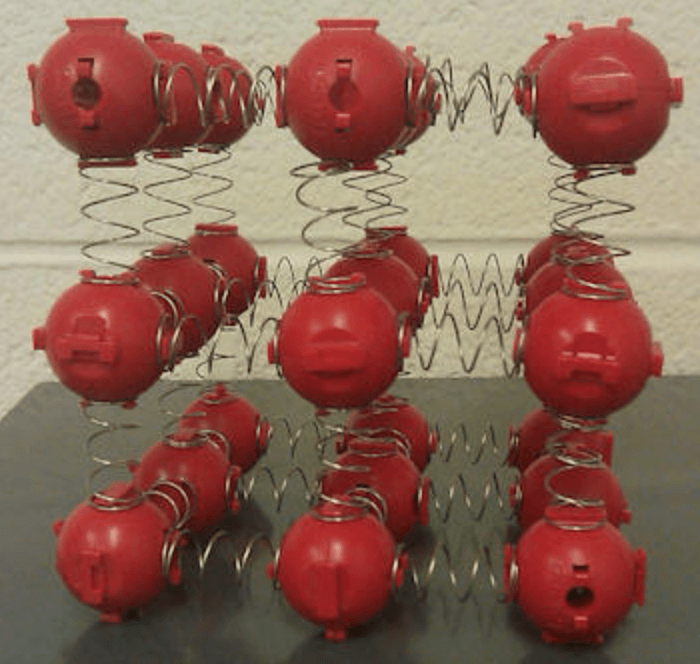 Take the image to the right for example, you give it a push and you are doing work against the frictional forces which in this case are present due to the springs between the particles, this transfers kinetic energy to the particles. These then collide with neighbouring atoms and increase the temperature and therefore the thermal energy of the system..
Take the image to the right for example, you give it a push and you are doing work against the frictional forces which in this case are present due to the springs between the particles, this transfers kinetic energy to the particles. These then collide with neighbouring atoms and increase the temperature and therefore the thermal energy of the system..
Doing work against any frictional forces results in the particles kinetic energy stores increasing, which ultimately raises the temperature of the room.
The next video is something you will hopefully have seen in class (otherwise you can prove it by doing it at home). Place a balloon over the top of a glass bottle, if the bottle is then placed in cold or room temperature water, nothing happens. If instead the bottle is placed in hot water, the thermal e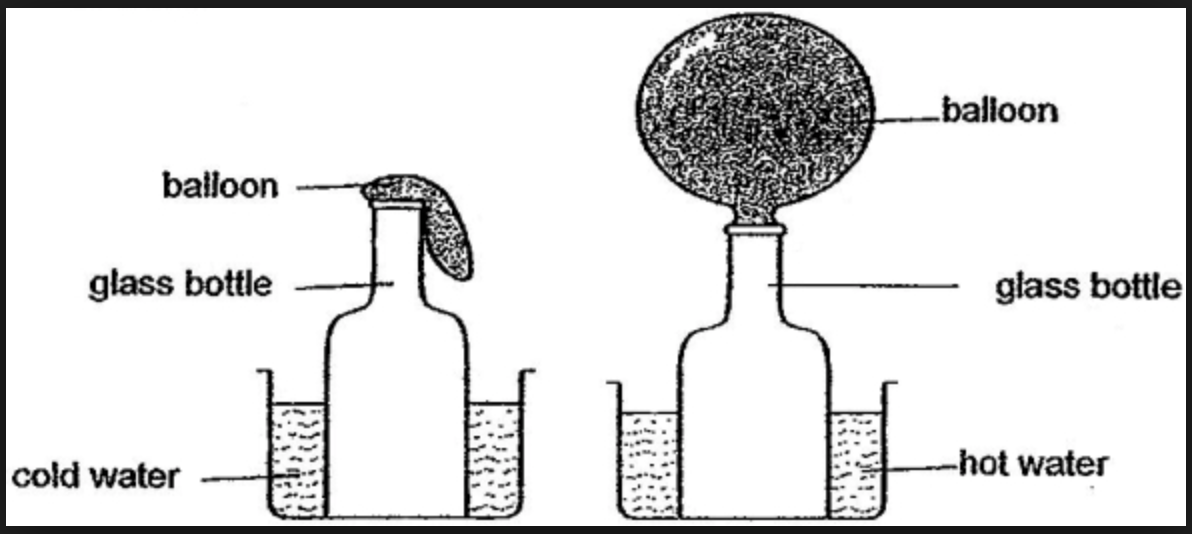 nergy conducts through the glass and to the air particles, this makes them heat up and increases their kinetic energy store. This results in the balloon inflating.
nergy conducts through the glass and to the air particles, this makes them heat up and increases their kinetic energy store. This results in the balloon inflating.
What do you think would happen if you kept swapping the balloon from the hot water to the cold water?
Would the balloon just keep inflating, then deflating and vice versa?
Using the knowledge learnt from above, you should now consider what the individual particles are doing as they flow into the balloon and then out of it again (as it keeps inflating and deflating). They will constantly be colliding with other gas particles and will inevitably increase their kinetic energy stores. In other words, if the process if repeated over and over, eventually the pure act of repetition will cause the balloon to eventually stay inflated due to the increase in the average kinetic energy. Watch the following video to see if this checks out:
The fact that work done on a gas can ultimately cause an increase in internal energy of a substance can be a real problem.
 What this page ultimately tells you is that the first page of this topic explaining the different types of energy is to some extent…. nonsense! There are not really ten different types of energy, really there are only two. One of them should be clear from the above, its kinetic, the idea the particles move. the other type of energy is known as potential energy which is a store of energy and this can be in different forms.
What this page ultimately tells you is that the first page of this topic explaining the different types of energy is to some extent…. nonsense! There are not really ten different types of energy, really there are only two. One of them should be clear from the above, its kinetic, the idea the particles move. the other type of energy is known as potential energy which is a store of energy and this can be in different forms.
Since work done is the definition of energy being transferred. It can be used to derive the equations for kinetic energy, gravitational potential energy and elastic potentials energy:
– Derivation



You must be logged in to post a comment.