Objectives:
- To understand that the amount of energy stored in or released from a system as its temperature changes can be calculated using the equation:
- To identify that the specific heat capacity of a substance is the amount of energy required to raise the temperature of one kilogram of the substance by one degree Celsius.
We have previously looked at the different types of energy stores and have also looked at some equations that are used to determine how much energy is in different energy types. These equations were:
But what about how much energy is takes to heat something up. Surely this is something that can be calculated since a kettle puts energy into water. If the amount of energy that is put in is measured, an equation may be able to be put together to calculate this figure too. This would then allow us to determine how much energy is required to say…
- heat our homes
- makes a cup of hot chocolate
- create a roast dinner on a Sunday
What the energy required to heat a substance up depends on:
- Which requires the most energy to heat up by
?
 The full glass requires the most energy, because there are more particles to raise the temperature by
The full glass requires the most energy, because there are more particles to raise the temperature by and so more overall energy required. So the mass is important.
- Which of the following requires the most energy to heat it up to
?
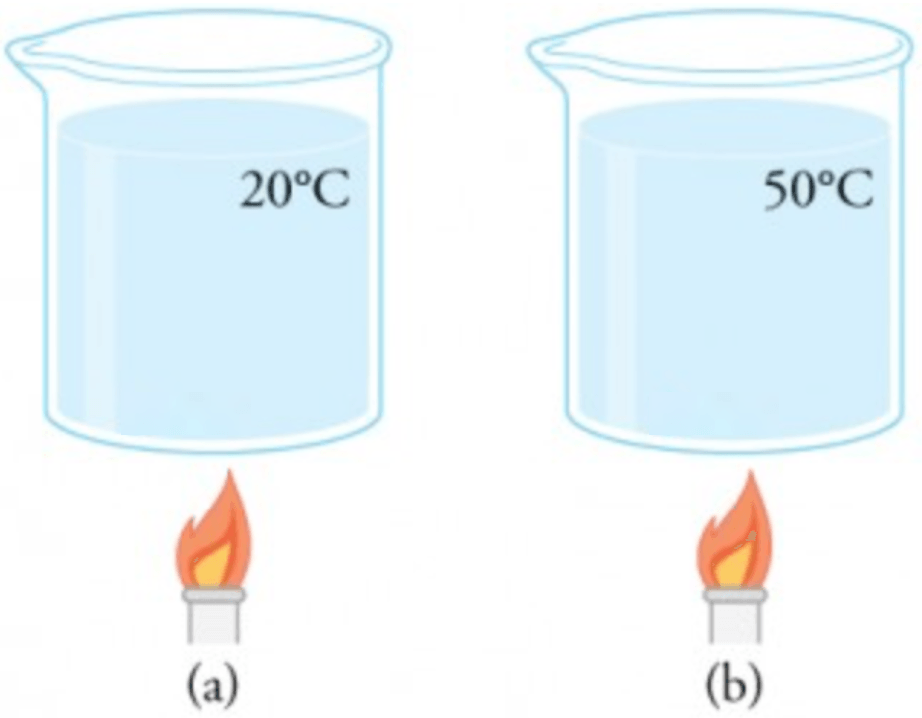 The beaker initially at
The beaker initially at requires more energy because each particle initially has less internal kinetic energy. So the temperature change is important.
- Which of the following, if both are initially at room temperature, requires the most energy to reach
?
 Both substances require different amounts of energy, hopefully we can all agree on that? If not, do a home experiment and put a certain amount of water in a saucepan and time how long it takes for it to reach
Both substances require different amounts of energy, hopefully we can all agree on that? If not, do a home experiment and put a certain amount of water in a saucepan and time how long it takes for it to reach , then do the same but with oil; you will notice that the oil heats up significantly faster if the same heating filament is used. This is because oil and water have different intermolecular bonds between then, have different densities and different types of particles etc, this property of a substance is called its heat capacity. Every substance has its own specific heat capacity value.
Putting this altogether:
The energy required to heat up a substance is (as was discussed above) dependent on the following three quantities:
- Mass
- the temperature change required
- the specific heat capacity
These quantities can be put together to form the equation:
where;
is the input energy given, measure in Joules,
is themes, measure in kilograms,
is the specific heat capacity of a substance, measure in Joules per kilogram per degrees celclius,
is the change in temperature, measure in degrees celcius,
The definition for specific heat capacity is the amount of energy required to raise the temperature of of a substance by
.
The reason for using a mass of and a temperature change of
is simply for simplicity. The equation above can be rearranged for specific heat capacity as is;
The units for specific heat capacity, as shown above, are something you will need to remember for you examinations. Personally I wouldn’t bother remembering the units but instead would remember how to find them; this is done by substituting the correct units into the equation for specific heat capacity;
You will be required to known how to practically determine the specific heat capacity of a substance. A method, risk assessment and set of results can be found on the following page:
Determining specific heat capacity through experiment
Determining the specific heat capacity of a substance
- How much energy is required to heat up 1.5 kg of water, which has a specific heat capacity of 4,181 J/kg℃, from 22 ℃ to 100 ℃?
- How much energy is required to heat 15g of oil, with a specific heat capacity of 1970 J/kg℃, from 22 ℃ to 299 ℃?
Putting the specific heat capacity to use
The following is a list of substances and their appropriate specific heat capacity values;
 Using this table try and answer the following questions:
Using this table try and answer the following questions:
- Which material requires the smallest amount of energy to raise the temperature of 1 kg by one degree? Why is this?
- You are tasked with making a radiator for someone who is not fussed about the expense;
- Which substance from the table above would be best to use to heat up the quickest and why?
- Which substance from the table above would be the best to use for it to retain its temperature for the longest period of time after the radiator was switched off, and why?
- What do you notice about the specific heat capacity values and the states of the matter of each?
- Water is renowned for its particularly high specific heat capacity value, why do you think helium has an even high value?
Specific heat capacity is useful when designing buildings, vehicles and clothing. In order to keep heat energy inside a building would you want to make the walls from a materials with a high or low specific heat capacity value and why?
The answer is a high value, this is because it will require more energy for it to heat up before it starts to release the energy to outside the building; additionally the wall will the retain the heat and release it slowly (for the same reason it absorbs it slowly), therefore it will take longer for it to release the energy to the outside of the building.
Here is a quick revision video on specific heat capacity:
Further reading:

You must be logged in to post a comment.