Objectives:
- To understand the electromagnetic spectrum and the properties of electromagnetic waves
- To learn the orders of magnitude of wavelengths the principal radiations from radio waviest gamma rays
We know that a wave is the transfer of energy from one place to another without transferring the particles itself; sound is an example of this, without the particles to transfer the energy, no sound would transmit from one location to another, sound is an example of a longitudinal wave. Light is an example of a transverse wave and can travel through one location to another without the need for particles to exist within that medium.
Light as we know it comes in a variety of different colours from red to violet.
The colours shown above are all part of the visible light spectrum which can be observed by the human eye, which is a region of a much larger spectrum known as the electromagnetic spectrum.
The Electromagnetic Spectrum
The electromagnetic spectrum is made up of seven regions, all of which have their uses due to their relative wavelengths.
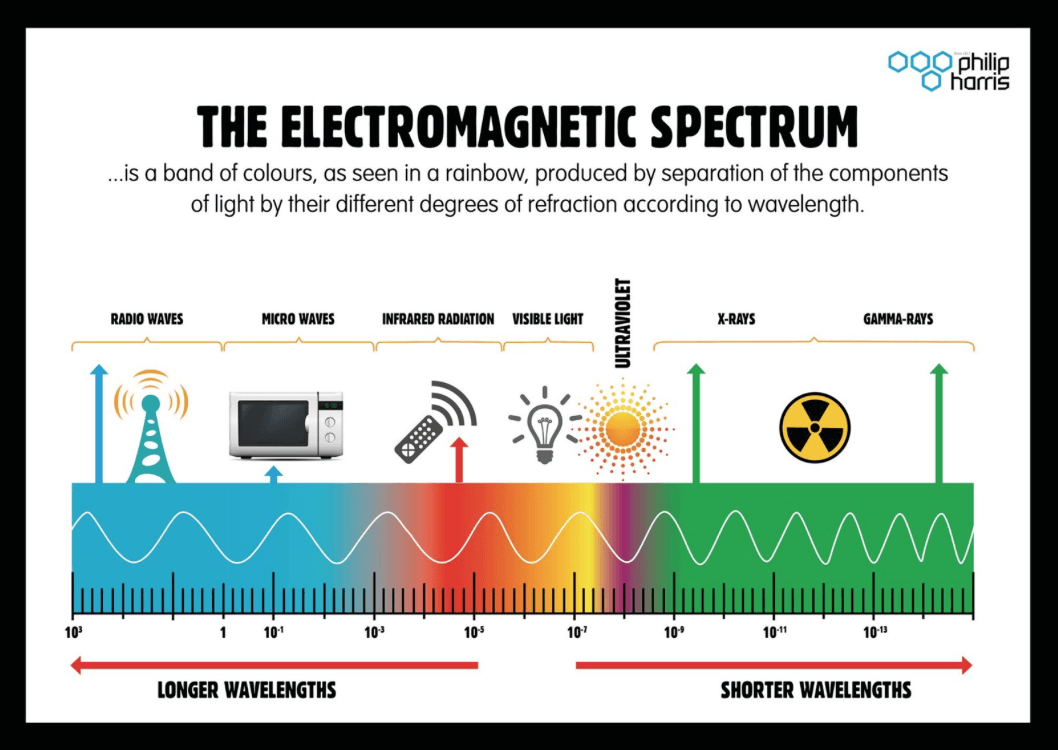 At A level you will be required to know all seven waves in the spectrum with their corresponding wavelengths, uses and dangers.
At A level you will be required to know all seven waves in the spectrum with their corresponding wavelengths, uses and dangers.
Each wave sits within a range of wavelengths, but for your examinations you should aim to recall the typical order of magnitude for each type of wave.
Radio waves:
Micro waves:
Infrared:
Visible light:
Ultraviolet:
X rays:
Gamma Rays:
There are no precise accepted boundaries between any adjacent portions so the ranges do overlap. The region of visible light does vary from person to person with the range extending from to
, but for your examinations the ranges from
are acceptable and more widely used in A level examinations.
X ray and gamma rays also tend to overlap as the key difference in the two of these is the method in which they are created. Gamma rays are formed in the process of radioactive decay, fission or fusion, whereas X rays are not.
At A level it typically helps you more to remember the typical wavelength of each wave in the EM spectrum rather than the frequency. However (and you will learn this in the Quantum Physics section), the amount of energy that any particular wave has is determined by its frequency not its wavelength. As discussed on the page discussing wave motion the speed of a wave is equal to the product of frequency and wavelength (), since
is a constant does it matter whether we define a wave by its frequency or wavelength?
The crucial aspect to not overrule in this case is the fact that all AM waves travel at the same speed in a vacuum. Since these waves can travel through different mediums and as such speed up or slow down, the wavelengths of these waves can change – but the frequency does not.
From the lowest frequency to the highest frequency they are:
- Radio waves (
);
- Microwaves (
);
- Infrared (
);
- Visible light (
);
- Ultraviolet (
);
- X-rays (
);
- Gamma rays (
)
Each of these waves have their own uses and dangers and are also produced using different methods – as outline below:
Further reading:
- Isaac Physics – Electromagnetic waves – This is a reading resource
- Research the discovery and early use of X-rays.
- Chernobyl: Crime without Punishment by Alla A. Yaroshinskaya – preview


You must be logged in to post a comment.