Objectives:
- To understand the photoelectric effect, including a simple experiment to demonstrate this effect
- To identify, understand and be able to explain a demonstration of the photoelectric effect, e.g. using a gold-leaf electroscope and zinc plate
- Acknowledge and appreciate the a one-to-one interaction between a photon and a surface electron
Previously you will have learned that light can travel in the form of waves or photons. The energy of a photon can be defined by the lights frequency;
 Evidence that light travels in the form of a particle
Evidence that light travels in the form of a particle
There are some examples that suggest that light can travel in the form of a particle, an example could be total internal reflection; light bouncing off a surface and maintaining all of its energy, but the idea light travelling in the form of a wave can also explain this. 
For us to acknowledge and accept that light can travel in the form of a particle we need proof – proof that the wave theory will not be able to explain.
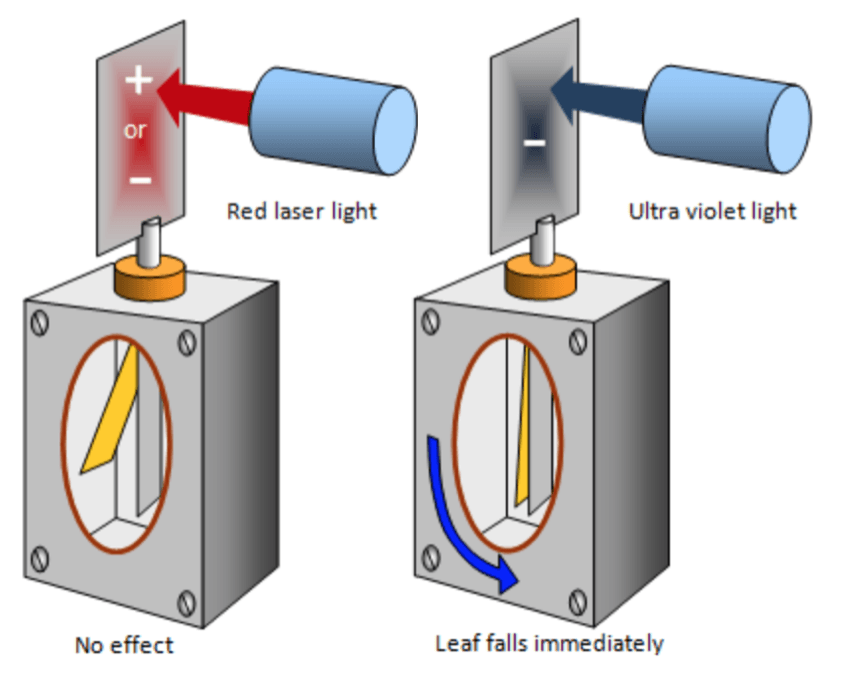
The photoelectric effect is a perfect example of how light can be thought of as a ‘quantum of energy’. It is the process whereby light is directed at a surface and the energy transfers into an electron. If the electron is given enough energy then it can escape the surface. In order to show this a gold leaf electroscope can be used (click here for an explanation as to how a gold leaf electroscope works);
If an electroscope has been charged then the gold leaf will deflect, this can be either be because the electroscope has become negatively charged (gained an excess amount of electrons), or because it is positively charged (lost some of its electrons).
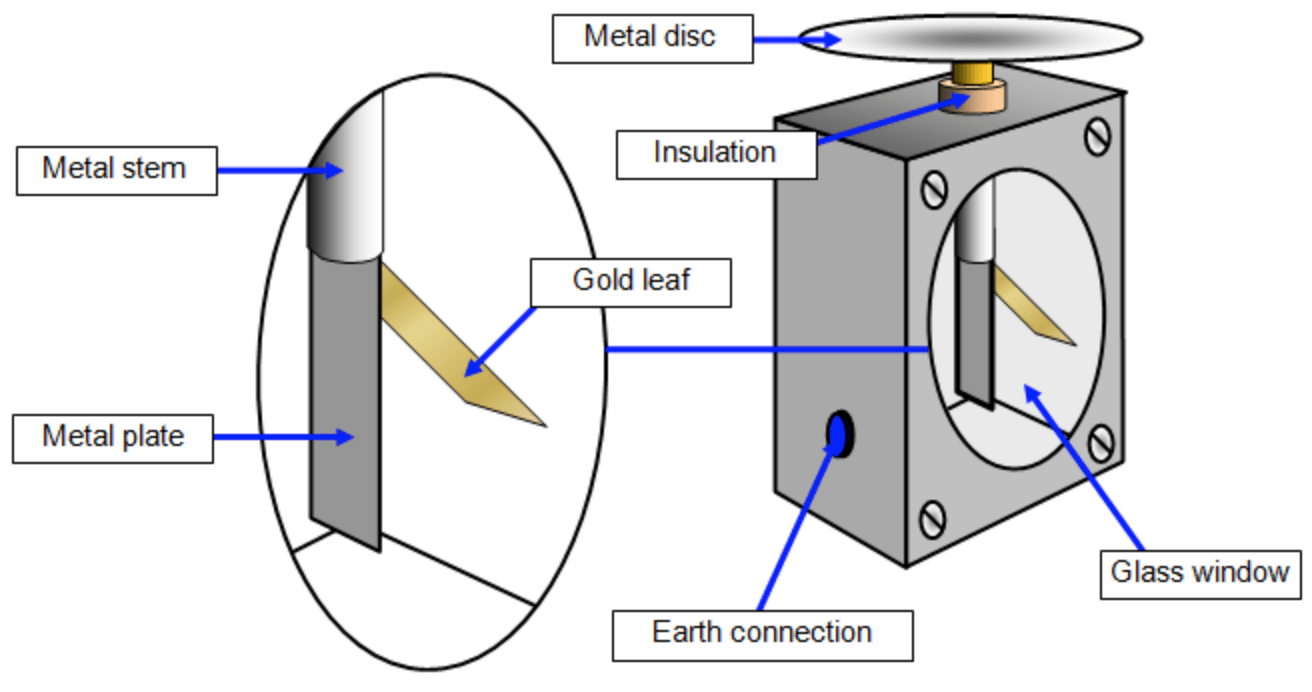
If red light is shone onto the the metal plate of the electroscope (using a lamp of some form), the plate will remain charged and the gold leaf will stay deflected. However, if the lamp was replaced by one emitting UV light, the electroscope can be seen to discharge.
It does not matter how long the red light shines on the metal plate, it does not discharge, however the UV light appears discharge the electroscope immediately. This suggests that the electrons in the plate require energy from a photon with a smaller wavelength (and therefore higher energy).
The observation that no matter how long the red light shines on the plate, no discharge is observed, provides evidence that light travels in the form of a particle and not a wave.
If the red light were travelling in the form of a wave, an electron could potentially absorb it. If the energy is not enough for it to escape the metal plate then surely it could just absorb a second photon so that it gain enough to become free, or maybe a third photon? Perhaps one thinks it is just unlikely that any electron will gain more than one photon? Eventually they would and the plate would discharge. Since this is not observed it is not what happens! The energy of the photons needs to be large enough for the electrons to escape.
If an electron absorbs a single photon of a high enough energy, that electron can escape – it would then be called a photoelectron (a photoelectron is exactly the same as an electron, but it has just undergone the photoelectric effect). This photoelectron will then leave the metal plate, the energy it then has would be in the form of kinetic energy. The following equation can then be written showing the conservation of energy:
where
is the energy of the photon
is the minimum energy required for an electron to escape the metal and is known as the work function.
is the kinetic energy of the photoelectron
Some electrons will have originated from further in the metal and therefore they will have a higher work function to overcome. Therefore the photoelectrons will have a maximum kinetic energy. Additionally, since the energy of a photon can be written as , we can complete the equation above to give:
Further reading:
- Isaac Physics – The photoelectric effect – This is a reading resource
- The Photoelectric Effect by Leigh Page – preview

You must be logged in to post a comment.