Objectives:
- To understand the particulate nature (photon model) of electromagnetic radiation
- To identify a photon as a quantum of energy of electromagnetic radiation
- To able to define the energy of a photon; $latex E = hf $ and
- To understand and use the electron volt (eV) as a unit of energy
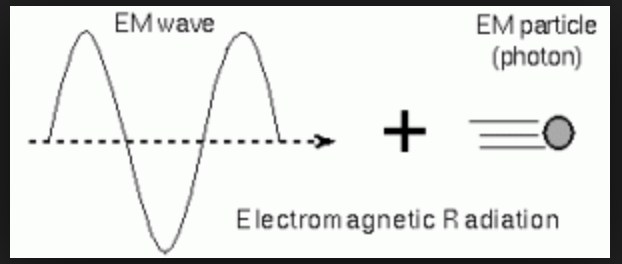
You will be aware that light travels in the form of electromagnetic waves, you may also have read or heard somewhere that light is made up of photons, which are considered particles.
Electromagnetic radiation travels in the form of transverse waves as seen by the fact that it can be reflected, refracted, diffracted or even polarised.
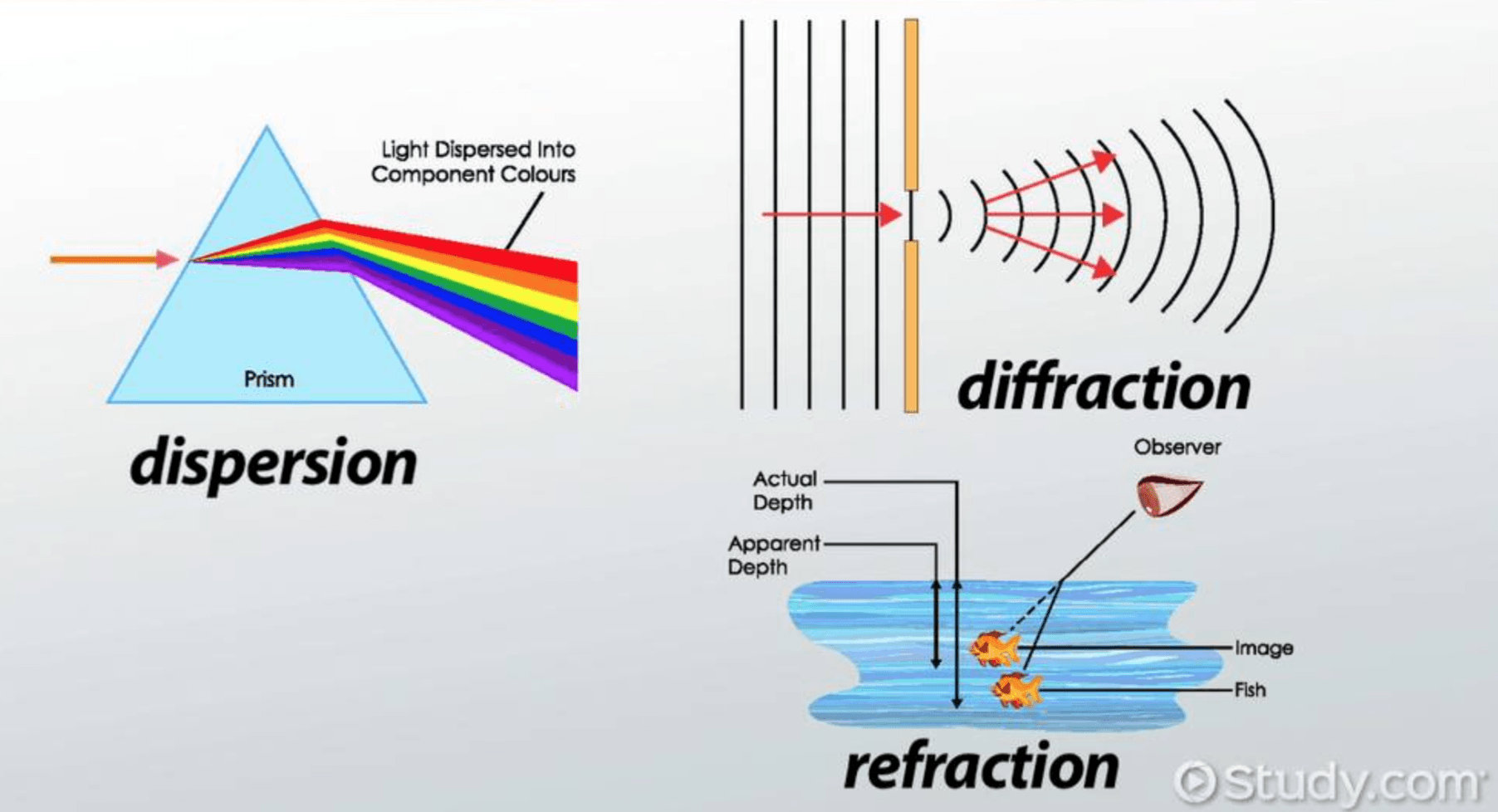
The idea that light could also be considered a particle should be acceptable; after-all particles reflect off surfaces, they can change direction when travelling through different mediums, they can even spread out when travelling through gaps, additionally if you refer back to the polarisation page and the analogy of a string oscillating in a certain plane, the vibrations could cease to exist if there is a slit in the wrong orientation to it that the wave has to pass through – in other word particles could potentially be polarised.
So does light travel in the form of a wave or a particle, or both?
Light in fact does have characteristics of both waves and particles and therefore it is stated that it travels in both forms at the same time – wave-particle duality.
Electromagnetic radiation is a packet of energy and if it travels in the form of a photon, the photon must be pure energy (because light doesn’t have any mass). A photon is therefore considered to be a ‘packet’ of energy, (but a very small amount of energy) known as a quantum of energy.
Energy of a Photon
Throughout your GCSE’s and A level so far you have come across a whole range of equations for energy;
You may notice that each of these equations involve physical quantities (not forms of radiation) – even involves the mass of a particle!
Light is defined by its frequency, since this is always constant no matter what medium it is in, so it makes sense that the energy of a photon is dependent on its frequency.
Consider the electromagnetic spectrum;
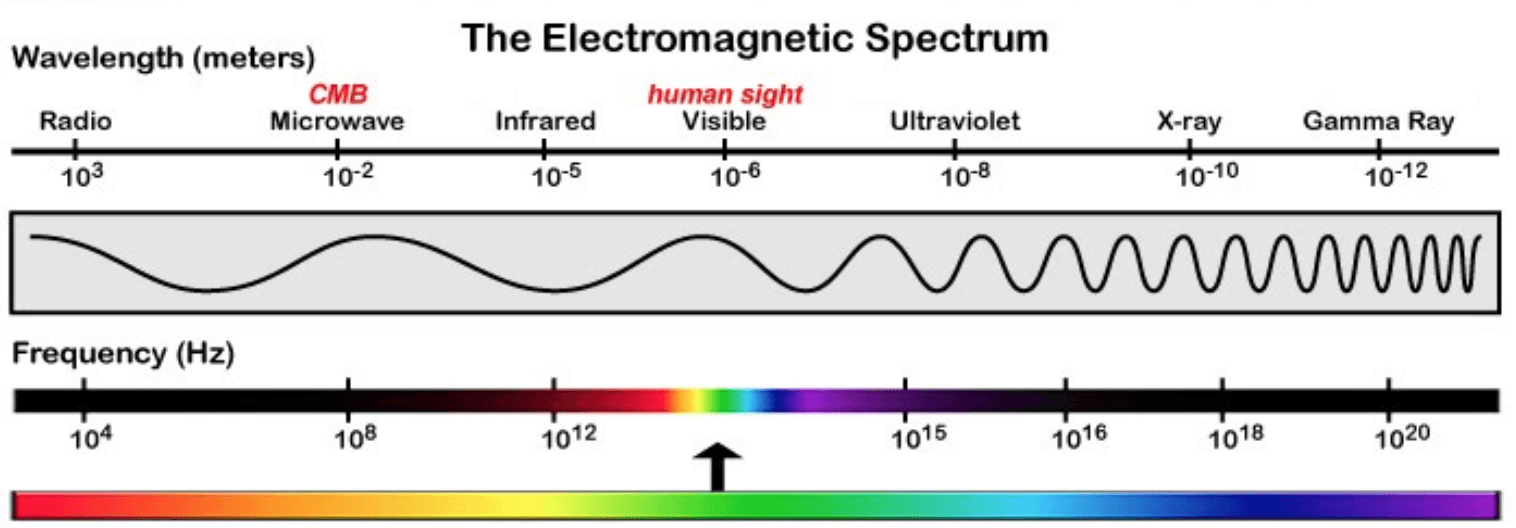
As shown in the EM spectrum, the frequency of radiation increases as the wavelength decreases (the closer to the gamma region the higher the frequency). Which of type of EM radiation has the most energy and which has the lowest? The answers are gamma rays and radio waves respectively as you will have known from your GCSE studies. So from this we can see that as frequency rises, the energy rises too.
The equation for the energy of a photon is;
where
is the energy of the photon
is a constant known as Planck’s constant
is the frequency of the wave or photon
By using and rearranging for
:
and subbing this into the the equation for the energy of a photon gives;
For waves that travel through a vacuum and hence at the speed of light (or in air since the speed is minimally different), this equation can be written as:
If the wavelength of EM radiation is known then is used, but if the frequency is known then you would use
.
Planck’s constant, is equal to
. The units of this constant can be determine from one of the equations with it in, e.g. rearranging
;
Example question: How much energy does a photon of red light with a wavelength have?
(given in the formula booklet)
If the same calculation if done for blue light with a wavelength of 475 nm, you can determine that the energy a photon would have is . This is larger than the energy a red photon has which is what you should expect.
These values for energy are tiny and so a more appropriate unit, known as the electronvolt is used – you should have come across it before, but below is a recap.
The Electron-Volt

If you read back through the notes on electromotive force and potential difference you will see that an equation was derived which contained . This is a really useful term in Physics and is labelled the electronvolt, it is a unit of energy that is used for particles that have a very small number of joules associated with it. For example, an electron travelling at 90% speed of light will have how much kinetic energy?
where
This is such a tiny amount of energy (and frequently smaller energies again are calculated for particles) that it is not always useful to refer to it in joules.
An electronvolt, , is an alternate unit for energy which is derived directly from the equation for the work done on charged particles,
;
If say an electron is accelerated through a potential difference of 1 Volt;
where
Hence the term electronvolt. Since work done is measured in joules, we can use this equation to determine how many joules are in 1 eV;
Further reading:
- The early history of quantum theory and the arguments over its interpretation are fascinating. Quantum: Einstein, Bohr, and the Great Debate about the Nature of Reality by Manjit Kumar explores them well (news article discussing it).
- The Photon’s Magnetic Field: Optical NMR Spectroscopy by Myron W. Evans – Preview
- There are a great bank of resources on energies in electronvolts available online.

You must be logged in to post a comment.