Objectives:
- Explain that electric current is a net flow of charged particles;
- Understand that current is the movement of electrons in metals and movement of ions in electrolytes;
- Explain what is meant by conventional current and electron flow.
What is electric current?
An electric current generally is the flow of electrons through a wire. Electrons are negatively charged and are free to flow between atoms in a wire, there are vast numbers of these electrons in any given volume of a metal and collectively they contribute towards a current in a wire.
The ions in a wire (or other solid structures) are fixed and so cannot flow. The electrons, when given energy to move all flow in one directions. Figure 1 displays these ions and electrons.

Traditionally (at GCSE) you are taught that current (the flow of electrons) flows from the positive terminal of a battery to the negative as shown:
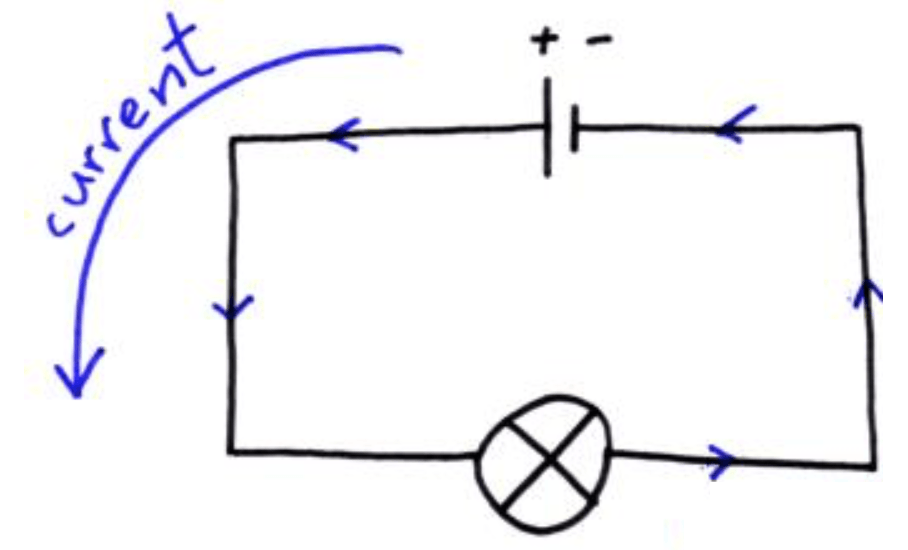
This in fact is not true as the electrons are repelled from the negative terminal and attracted to the positive terminal of a battery. The direction of current that you are familiar with is known as conventional current and is the direction that positively charged particles would flow in. However, since it is electrons that flow in a wire, which are negatively charged (the direction has to take into account the sign (+/-) of the charge) the direction of electron flow should be noted:
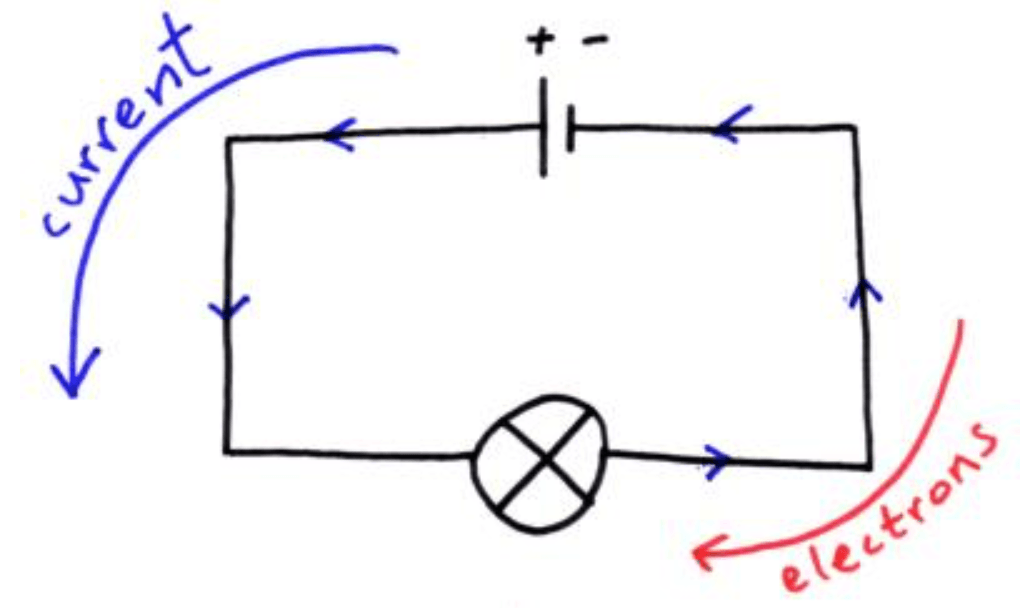
In the diagram above the direction of the electrons shows exactly as it suggests, the direction of current flow (also known as conventional current) is the direction that we assume positively charged particles to be moving in (if we weren’t to know it was electrons that were moving).
Here is a video which may help to explain things in a different way:
The electron
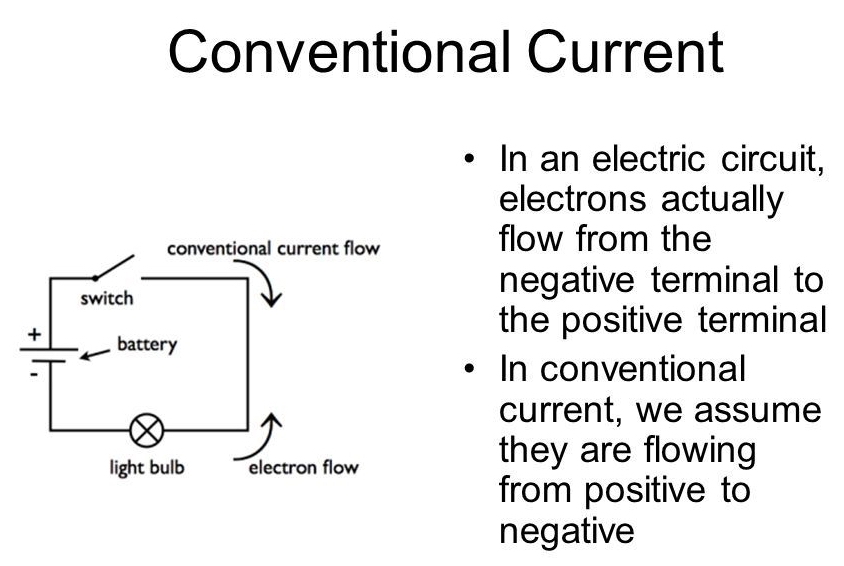
When electricity was discovered scientists tried many experiments to find out which way the electricity was flowing around circuits, but they struggled to find the direction of the flow.
They knew there were two types of electric charge, positive and negative, and they decided to say that electricity was a flow of positive charge from positive to negative. Although this was a guess, a decision had to be made!
The electron was discovered in 1897 and it was found to have a negative charge. The guess made in the early days of electricity was wrong! Electricity in almost all conductors is really the flow of electrons (negative charge) from negative to positive.
By the time the electron was discovered the idea of electricity flowing from positive to negative (conventional current) was firmly established. Luckily it is not a problem to think of electricity in this way because positive charge flowing forwards is equivalent to negative charge flowing backwards.
To prevent confusion you should always use conventional current when trying to understand how circuits work, imagine positively charged particles flowing from positive to negative.
Why is conventional current still used?
Every year somebody asks (and quite rightly), why we still use conventional current when it is in fact electrons that flow in wires and not positively charged particles.
The reason becomes more clear whilst progressing through A-Level physics but for now, think back to you GCSE chemistry and to electrolysis:
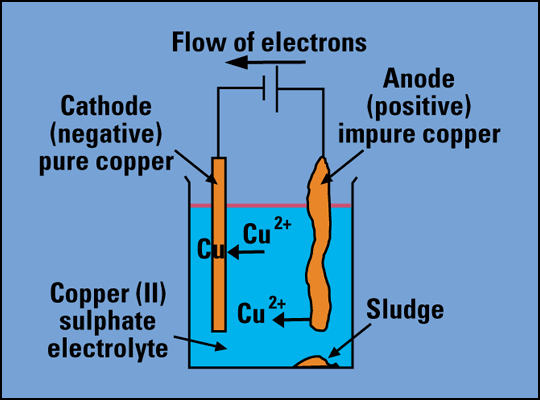
If you look inside the solution in the above image, you can see that it is Cu(2+) atoms that are moving. These are positively charged particles, so in this case conventional current is the direction in which the positively charged particles are flowing. Positive particles, because they are more absent of electrons than they are protons (this is material from GCSE but will be covered in module 6) are known as ions, therefore the particles moving in electrolysis are ions.
Does the state of matter change whether the material can conduct electricity or not?
Conductors contain free electrons and conduct electricity because these electrons are free to flow through the material. Insulators do not contain many (or any at all) free electrons and as a result cannot allow a current to flow – thereby restricting any current from flowing.

Further reading:
- Isaac Physics – Electric Current – This is a reading resource
- Research older circuit symbols and compare them with those of today, commenting on which they prefer and why.
- Children of Light: How Electrification Changed Britain Forever by Gavin Weightman (Can be found on Amazon).


You must be logged in to post a comment.